One issue that is always at the top of world concerns is the environment and the consequences of human activities on it. The prevention of major evils is a subject that has been on the world’s agendas for several decades.
The climate crisis is one of the greatest threats facing humanity today. The increase in greenhouse gas emissions, deforestation, loss of biodiversity and scarcity of natural resources are challenges that directly affect our planet and put at risk the well-being of future generations.
Recently, an effort has been made to combat the effects already made to the environment. In this article, I want to give attention to road transport, with the invention of new ways of propulsion from electric to hydrogen, these being the most advanced.
However, just around the corner, research is being carried out into a new alternative, which in itself would revolutionise more than the car market, it would revolutionise the transport business for an entire one.
At Cambridge University, researchers are using the power of photosynthesis to convert CO2, water and solar radiation into multi-carbon fuels such as ethanol and propanol.
The main ambition is to create a circular economy by synthesising high energy density liquids through CO2 and H2O enhanced by solar radiation.
The major problem they encountered was that despite the progress already made producing simple gaseous products, the construction of unassisted photoelectrochemical devices for multi-carbon liquid production remains a major challenge.
(To find out what these devices are and why they were used for this production, click here)
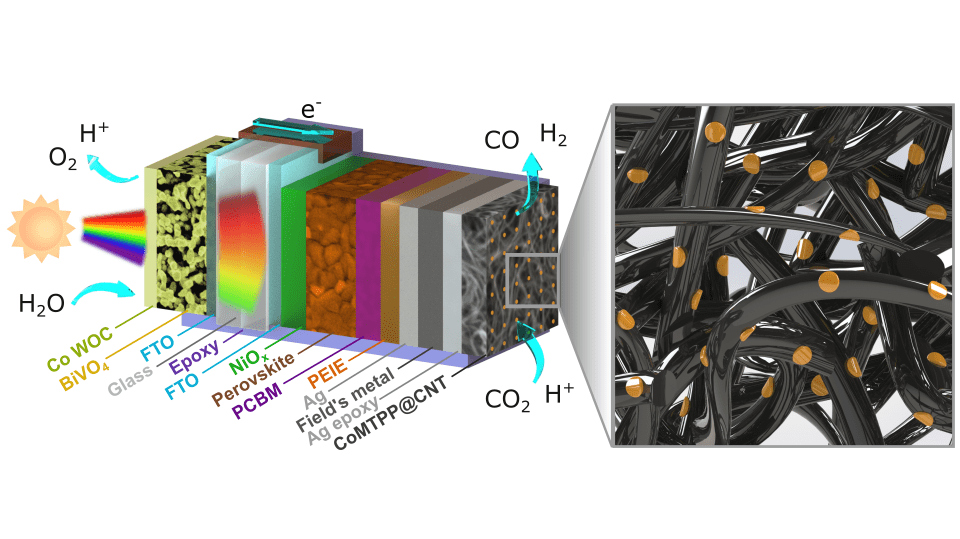
With this in mind, the researchers stayed with their goals and carried out the work. In this way, they collected artificial leaf devices by integrating an oxide-derived electrocatalyst, Cu₉₄Pd₆, with tandem* perovskite-BiVO4 light absorbers. This device is capable of coupling CO2 with water oxidation, allowing the production of multi-carbon alcohols.
(To find out what a Cu₉₄Pd₆ oxide-derived electrocatalyst and a perovskite-BiVO4 tandem light absorber is, click here)
The researchers mention two important aspects of the devices. First, the Cu₉₄Pd₆ | perovskite-BiVO4 tandem binding device demonstrates a Faradaic efficiency of approximately 7.5% for the production of multi-carbon alcohols, with the ratio of ethanol and n-propanol being equal (1:1).
The second aspect that the researchers mention, concerns the autonomous wireless device that produces alcohols at a concentration of approximately 1 μmol/cm² after 20 hours of unattended operation. This device operates under solar exposure conditions with an irradiance of 1.5G and a production frequency of 40 μmol/h/g_(Cu₉₄Pd₆).
As such, this paper discusses the development of an advanced artificial leaf technology that uses solar energy to produce multi-carbon liquid fuel. Furthermore, the efficiency and operational characteristics of the device are mentioned, which may indicate advances in sustainable fuel production using solar technologies.
This work arrives at multi-carbon liquid fuels from CO₂ using an artificial sheet. This means that the technology developed in this study allows carbon dioxide to be directly converted into complex liquid fuels using solar energy as an energy source. The ability to generate value-added products from sunlight represents a significant advance towards the sustainable and efficient use of solar energy in fuel production. The approach worked out can have important implications for the development of cleaner energy systems and the reduction of greenhouse gas emissions, contributing to the mitigation of climate change.
This work has captivated my interest as it combines two areas of interest to me, renewable energy and research into new sustainable alternatives. I will continue to keep an eye on the work of these researchers to keep abreast of the results they obtain.
Definition
*a long bicycle with two seats and two sets of pedals, one behind the other; in this context, it refers to a layered configuration where perovskite and BiVO4 are combined in a solar cell to more efficiently harness incident sunlight;
- Unassisted photoelectrochemical devices for liquid multi-carbon production
Unassisted photoelectrochemical devices for the liquid production of multi-carbon refers to systems that use the energy of sunlight to catalyse chemical reactions that result in the formation of organic compounds containing multiple carbon atoms (multi-carbon) from simple raw materials such as carbon dioxide (CO2) and water (H2O).
These devices combine the principles of photocatalysis (using sunlight to promote chemical reactions) and electrocatalysis (using electrodes to facilitate electrochemical reactions) to convert solar energy into stored chemical energy in the organic compounds produced.
These devices are of great interest in sustainable energy research as they have the potential to convert CO2, a major greenhouse gas, into valuable chemicals and renewable fuels. However, the efficiency and selectivity of these devices are still challenges to overcome, and research is underway to optimise the materials and processes involved in these photoelectrochemical reactions.
2. Perovskite – BiVO4
It corresponds to a hybrid structure that combines the perovskite material with the BiVO4 compound.
Perovskite is a type of crystalline structure named after its discovery in a mineral called perovskite. It has a general chemical composition of ABX3, where A and B are metal ions and X is an anion. The perovskite structure is widely studied and used in various applications, including solar energy, photocatalysis and electronic devices.
On the other hand, BiVO4 is a semiconductor oxide composed of bismuth (Bi) and vanadium (V), which has photocatalytic properties. BiVO4 is known for its ability to absorb sunlight and use this energy to promote chemical reactions, such as the decomposition of water or the reduction of pollutants.
Therefore, the combination of perovskite with BiVO4, known as perovskite-BiVO4, is an approach that seeks to combine the unique properties of perovskite with the photocatalytic properties of BiVO4. This may have promising applications in photovoltaic devices, solar cells and other technologies related to solar energy conversion.
3. Perovskite – BiVO4 tandem light absorbers
They refer to a layered configuration where perovskite and BiVO4 are combined in a solar cell to more efficiently harness incident sunlight.
In this tandem arrangement, the perovskite is responsible for absorbing high-energy light (such as blue light) and converting that energy into electricity, while the BiVO4 is responsible for absorbing low-energy light (such as red light) and converting it into electricity. This combination allows a wider range of the solar spectrum to be captured and converted into electricity, thus increasing the overall efficiency of the solar cell.
The layered structure allows the perovskite and BiVO4 to act together, each absorbing a specific part of the solar spectrum, thus improving the efficiency of the photovoltaic conversion. This tandem configuration is a promising strategy to overcome the limitations of individual materials and improve the efficiency and performance of solar cells.
4. Oxide-derived Cu₉₄Pd₆ electrocatalyst
It is a type of electrocatalysis composed of an alloy of copper (Cu) and palladium (Pd) that is derived from an oxide. Specifically, the term refers to an electrocatalyst obtained from the reduction of a precursor oxide containing copper and palladium.
The oxide derivation technique is used to synthesise highly efficient catalytic materials with improved properties. It consists of heating the precursor oxide to high temperatures in a controlled environment to allow the chemical reduction reaction to take place, resulting in the formation of a metal alloy.
In this case, the compound is mainly copper (Cu), in a proportion of 94%, then there is palladium (Pd), in a proportion of 6%. This oxide-derived metal alloy possesses specific catalytic properties that can be exploited in electrochemical reactions, such as the electrocatalysis of reactions involving energy conversion, such as oxidation and reduction of chemical species.
These oxide-derived electrocatalysts have the advantage of combining the catalytic activity of the metal with the stability and controlled structure of the precursor oxide, which can result in enhanced performance compared to conventional catalysts. These materials are widely studied for various applications such as fuel cells, water electrolysis, energy storage and other technologies related to electrochemical energy conversion.
References
Rahaman, M., Andrei, V., Wright, D., Lam, E., Pornrungroj, C., Bhattacharjee, S., Pichler, C. M. , Greer, H. F., Baumberg, J. J., Reisner, E., 2023. Solar-driven liquid multi-carbon fuel production using a standalone perovskite–BiVO4 artificial leaf | Request PDF. Available from: https://www.researchgate.net/publication/370869256_Solar-driven_liquid_multi-carbon_fuel_production_using_a_standalone_perovskite-BiVO4_artificial_leaf [accessed Jun 08 2023].




Leave a comment